Pioneering simulation methods should aid device development and evaluation, and could eventually lead to personalized devices.
For many Americans, former President Bill Clinton’s recent heart procedure was a lesson in the miracle of modern medicine. Six years after undergoing quadruple bypass surgery, one of the four bypass grafts Clinton received had become completely blocked. The solution seemed simple: After being admitted to a New York hospital for chest pain, Clinton underwent an hour-long surgery and walked away with two new stents that propped his artery open.
Once considered a miracle, stent installations like the one performed on Clinton today are viewed as low-risk procedures. In the U.S. alone, more than one million patients with heart disease receive stent implants every year. While stents save lives, side effects such as blood clots and heart attacks remain fairly common. But research in this area is complicated by the complexities of the human arterial system: There are simply too many variables and too much data to sift through.
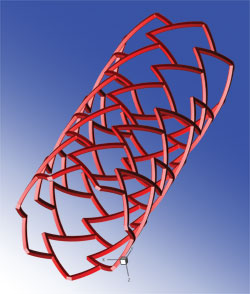
A fully expanded 3D model of a stent with a repeated “delta-wing” shape cell design. Image created in Tecplot 360 by Elazer Edelman, Vijaya Kolachalama, and Evan Levine.
A pioneer in stent research, Dr. Elazer R. Edelman, Professor of Health Sciences and Technology at Massachusetts Institute of Technology (MIT) and Professor of Medicine at Harvard Medical School, is working to improve stents along with Dr. Vijaya B. Kolachalama, Postdoctoral Associate at the Edelman Laboratory at MIT. Together, these researchers are helping to revolutionize the world of heart therapy using computational fluid dynamics (CFD) modeling, bench-top experiments, animal modeling, and visualization.
Specifically, Drs. Edelman and Kolachalama use CFD modeling and visualization to study how stents, as well as drugs delivered from stents can treat arteries on the one hand and cause blood clots on the other. They also hope to use it to predict whether specific arteries with stent implants are at risk of blood clots. “If we can accurately predict problems,” says Dr. Edelman. “We can help clinicians determine when to be more vigilant with patients about medications or additional procedures.”
The results could help medical device companies develop safer, more effective stents and aid the in the regulatory approval process. Their work ultimately could lead to the ability to design individualized stents for patients.
Using the Tecplot 360 CFD visualization tool, Dr. Edelman and Dr. Kolachalama successfully generated visuals that showed them how the artery and drugs would behave under different conditions…
Try Tecplot 360 for Free
Today’s innovative stent technology
To understand the researchers’ work, consider how doctors treat heart patients. To reopen a blocked artery, physicians inflate a balloon at the end of a catheter inserted into the patient’s artery to compact atherosclerotic plaque against the artery walls, a procedure known as angioplasty. Until recently, physicians would then install a small metal-mesh tube called a “stent” to keep the plaque from snapping back into the artery. Stents prevent this recoil, but tissue grows over the stent as part of the healing response. In 25 to 50 percent of cases, the tissue growth is so much that flow through the artery is once again insufficient and another procedure is required.
To prevent the re-blocking, scientists started coating the stents with drugs, often imbedded in a thin polymer material for time release. Called “drug-eluting stents,” these implants have reduced the need for repeat procedures to less than 10 percent. Yet, even though the drug-eluting stents prevented tissue and plaque blockage, they created new and different issues for a small percentage of patients, resulting in life-threatening side effects like blood clots and heart attacks.
This problem lies with the way the metal mesh stents must sit in the artery where they deliver their drugs. The stents lie against the wall of the artery but still protrude into the artery lumen. The mesh-like structure of the stent creates alternating flow disruptions much like rocks create white water in a flowing stream. Drug comes off the mesh struts at high concentrations and, once released, is now subject to areas of high and low flow. The deposition and distribution of the drug within the artery wall is therefore non-uniform and, though governed by classic flow equations, is increasingly non-intuitive and virtually impossible to predict. This issue becomes even more complex to understand when stents are placed at locations such as arterial branch points or tortuous vessels. It is now believed that these heterogeneous drug distribution patterns, with areas of extremely high drug concentration alternating with areas virtually depleted of drug, contribute to localization of blood clots.
Simulating complex results through CFD modeling and visualization
Observing that the chance of blood clots rises along with the amount of drug delivered, Dr. Edelman and Dr. Kolachalama realized they needed an effective method for identifying and predicting drug delivery patterns from stents in complex arterial vessels.
Human trials and animal experiments provide valuable data related to the biologic response to stents and drugs. Yet, the researchers could not examine the issues of drug distribution variability from these studies alone. Modern imaging technology cannot provide a complete view of the drug patterns and patient variability made it impossible to track the large number of variables at play. Even subtle differences in the geometry of arteries or native disease, amount of drug delivered, change in drug absorption in different parts of the artery, or the way the stent is implanted can have dramatic effects in drug uptake.
Drs. Edelman and Kolachalama did use what they had learned from clinical experience and animal models, as well as bench-top studies, to create physiologically relevant fluid dynamic models – using the best of both worlds. The animal and human data served as right input conditions to the CFD models, and the power of these tools was fully utilized by testing multiple variations in precisely the same manner repeatedly.
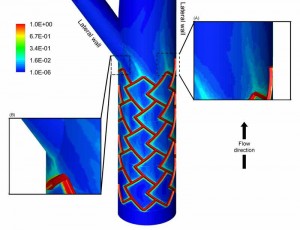
This image shows the surface contour map of drug concentration in a bifurcating (branching) artery. Drug is released from the surfaces of the stent where red color indicates regions of more drug and blue indicates low drug concentration. The MIT researchers predicted that the complex pattern of the drug distribution depended on many factors including the blood flow that enters the bifurcation (branch) and the nature with which it splits at the fork. The insets show high magnification images of the drug pattern (A) on the lateral wall of the main branch and (B) near the region where the flow divides at the bifurcation. Tecplot 360 image created by Elazer Edelman, Vijaya Kolachalama, and Evan Levine.
In their view, this was the ultimate use of CFD modeling, a branch of fluid mechanics that uses complex algorithms to analyze problems that involve fluid flows. The goal was to simulate what would happen in an artery under multiple complex but physiologically relevant conditions. “It is precisely here that CFD modeling and visualization is ideal,” says Dr. Edelman. “We have helped people understand that CFD is a perfect tool for integrating physiologic data and complex non-intuitive conditions to answer important questions in medicine.”The researchers began their work by gathering massive amounts of biometric data from several sources and in many formats to create mathematical models that would then be solved against multiple variables or parameters.
Visuals that Show How the Artery and Drugs Behave
The simulation yields numerically formatted results, however, making it difficult or impossible for the human brain to interpret. This is where visualization comes in. Using the Tecplot 360 CFD visualization tool, Dr. Edelman and Dr. Kolachalama successfully generated visuals that showed them how the artery and drugs would behave under different conditions — similar to looking at the visual results from an X-ray or video scope. The resulting images gave them insight into how drugs deposited from a stent are affected by the positioning of the stent, changes in blood flow where arteries meet, and blood flow changes created by the stent itself.
“By observing the arterial drug distribution patterns for various settings, we understood that the drug released from the stent does not reach uniformly to all regions of the vessel; and this non-uniformity depends on where the stent is placed in the artery as well as the blood flow that is entering the vessel,” Dr. Edelman says. “Appreciating this phenomenon for more complex cases like branched vessels is non-intuitive, but we now have a computer model that gives us the much needed insight.”
Improving the safety and effectiveness of stent implants
These developments are just the beginning, according to Dr. Edelman and Dr. Kolachalama. In the long run, the two researchers expect CFD modeling and visualization to improve both the safety and effectiveness of stent implants. “We as scientists now have the means to study stent placement in arteries in a manner not possible before,” says Dr. Edelman. “This has tremendous implications for engineers as they develop new devices designed to reduce the complications.”
In addition, Dr. Edelman and Dr. Kolachalama believe CFD modeling and visualization will help to streamline the regulatory process by giving regulatory agencies the information they need to determine whether proposed changes to stent technology are large enough to require a new clinical trial. “Today, these agencies are in a no-win situation,” says Dr. Edelman. “If they push through a new technology that has a problem, they are criticized for caring more about the technology than the patient. But if they establish barriers that are too high, then critical technologies become entrapped in an endless process of regulatory scrutiny and never make it into the clinic — criticized now for not caring enough about patients to provide them with the technology they need.”
Ultimately, Dr. Edelman and Dr. Kolachalama expect CFD modeling and visualization will assist physicians in making better in-the-moment decisions for their patients. With different artery scenarios for each patient, scores of stent types and brands, and short timelines with which to make critical, life-saving decisions, doctors will be able to sort through mountains of data to select the best stent for a particular patient – based on fact rather than perception.
“The era of intuition is over,” Dr. Edelman says. “The pioneers in this area were capable of intuiting what something should look like. But today there are just too many factors at play to rely on one’s past experience and predictive instinct.”
With Clinton, doctors used the newer drug-eluting stents, likely basing their choice on what they could observe about the former president’s arterial structure along with their knowledge of different stent brands. Just as doctors gave Clinton an excellent prognosis, in the future, stent implants free of complications will likely become the rule.
The Tecplot CFD visualization advantage
CFD modeling and visualization, Dr. Edelman and Dr. Kolachalama believe, will eventually allow doctors to use computers to compile all the options available, input them along with a patient’s medical data into a virtual artery and – like a GPS system that optimizes a driver’s route – sort through all the variables to determine which stent is optimal for that patient as they are doing the procedure.
“The day will come when an image is obtained, the doctor can directly interact with the computer by saying, ‘please optimize stent intervention,’ and the computer will select the appropriate optimization for each individual patient,” says Dr. Kolachalama. “Thanks to CFD modeling and visualization, it could be that accurate and that efficient, resulting in an even better outcome for the patient.”




